What Does K Mean In A Relationship . if you're seeing this message, it means we're having trouble loading external resources on our website. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. — what is the relationship between \(k_1\), \(k_2\), and \(k_3\), all at 100°c? — the acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. there is a simple relationship between the magnitude of \(k_a\) for an acid and \(k_b\) for its conjugate base. — the ph scale is the most familiar measure of acidity and basicity, but pka, pkb, ka, and kb are better for. the equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical. The expression for \(k_1\) has \([no]^2\) in. If you're behind a web filter,.
from 365datascience.com
— what is the relationship between \(k_1\), \(k_2\), and \(k_3\), all at 100°c? If you're behind a web filter,. if you're seeing this message, it means we're having trouble loading external resources on our website. the equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical. there is a simple relationship between the magnitude of \(k_a\) for an acid and \(k_b\) for its conjugate base. — the acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. — the ph scale is the most familiar measure of acidity and basicity, but pka, pkb, ka, and kb are better for. The expression for \(k_1\) has \([no]^2\) in.
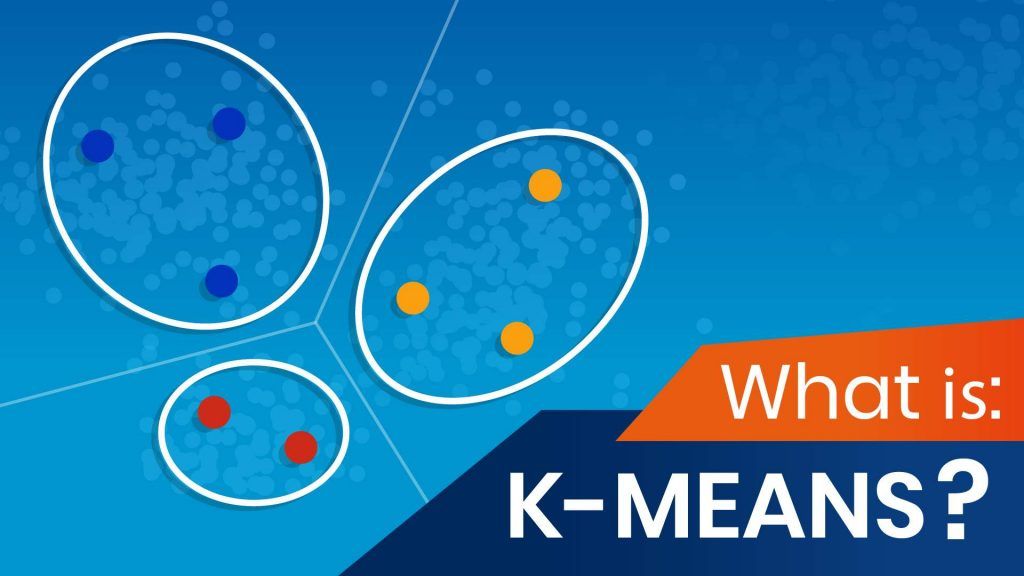
What Is Kmeans Clustering? 365 Data Science
What Does K Mean In A Relationship there is a simple relationship between the magnitude of \(k_a\) for an acid and \(k_b\) for its conjugate base. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. — the acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. If you're behind a web filter,. — the ph scale is the most familiar measure of acidity and basicity, but pka, pkb, ka, and kb are better for. there is a simple relationship between the magnitude of \(k_a\) for an acid and \(k_b\) for its conjugate base. if you're seeing this message, it means we're having trouble loading external resources on our website. the equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical. The expression for \(k_1\) has \([no]^2\) in. — what is the relationship between \(k_1\), \(k_2\), and \(k_3\), all at 100°c?
From www.datanovia.com
Visualisation du Clustering KMeans dans R Guide Etape par Etape What Does K Mean In A Relationship the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. The expression for \(k_1\) has \([no]^2\) in. if you're seeing this message, it means we're having trouble loading external resources on our website. the equilibrium constant of a chemical reaction is the value of its reaction quotient at. What Does K Mean In A Relationship.
From www.postnetwork.co
KMeans Clustering Algorithm in Machine Learning Academy What Does K Mean In A Relationship the equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical. The expression for \(k_1\) has \([no]^2\) in. If you're behind a web filter,. — the acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k. What Does K Mean In A Relationship.
From exorwrrag.blob.core.windows.net
What Does K Mean In Electricity at Wilma Plank blog What Does K Mean In A Relationship there is a simple relationship between the magnitude of \(k_a\) for an acid and \(k_b\) for its conjugate base. — the ph scale is the most familiar measure of acidity and basicity, but pka, pkb, ka, and kb are better for. If you're behind a web filter,. — the acid dissociation constant is the equilibrium constant of. What Does K Mean In A Relationship.
From www.codingninjas.com
Kmean Algorithm Coding Ninjas What Does K Mean In A Relationship If you're behind a web filter,. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. if you're seeing this message, it means we're having trouble loading external resources on our website. — the ph scale is the most familiar measure of acidity and basicity, but pka, pkb,. What Does K Mean In A Relationship.
From blog.finxter.com
The KMeans Algorithm in Python Be on the Right Side of Change What Does K Mean In A Relationship — the ph scale is the most familiar measure of acidity and basicity, but pka, pkb, ka, and kb are better for. — the acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. — what is the relationship between \(k_1\), \(k_2\), and \(k_3\), all at 100°c?. What Does K Mean In A Relationship.
From slideplayer.com
Chapter 15 Chemical Equilibrium ppt download What Does K Mean In A Relationship if you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter,. — the ph scale is the most familiar measure of acidity and basicity, but pka, pkb, ka, and kb are better for. there is a simple relationship between the magnitude of \(k_a\) for an acid. What Does K Mean In A Relationship.
From www.researchgate.net
Kmeans clustering of differentially expressed proteins across three What Does K Mean In A Relationship if you're seeing this message, it means we're having trouble loading external resources on our website. — what is the relationship between \(k_1\), \(k_2\), and \(k_3\), all at 100°c? If you're behind a web filter,. the equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a. What Does K Mean In A Relationship.
From exorwrrag.blob.core.windows.net
What Does K Mean In Electricity at Wilma Plank blog What Does K Mean In A Relationship If you're behind a web filter,. — the ph scale is the most familiar measure of acidity and basicity, but pka, pkb, ka, and kb are better for. — what is the relationship between \(k_1\), \(k_2\), and \(k_3\), all at 100°c? The expression for \(k_1\) has \([no]^2\) in. — the acid dissociation constant is the equilibrium constant. What Does K Mean In A Relationship.
From nlp.stanford.edu
Kmeans What Does K Mean In A Relationship there is a simple relationship between the magnitude of \(k_a\) for an acid and \(k_b\) for its conjugate base. The expression for \(k_1\) has \([no]^2\) in. If you're behind a web filter,. — the acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. if you're seeing. What Does K Mean In A Relationship.
From whatmeaninger.blogspot.com
What Does K Mean In Money What Does Meaning What Does K Mean In A Relationship the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. if you're seeing this message, it means we're having trouble loading external resources on our website. — what is the relationship between \(k_1\), \(k_2\), and \(k_3\), all at 100°c? — the ph scale is the most familiar. What Does K Mean In A Relationship.
From www.researchgate.net
Flow Diagram of KMean Algorithm Download Scientific Diagram What Does K Mean In A Relationship — what is the relationship between \(k_1\), \(k_2\), and \(k_3\), all at 100°c? the equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical. — the acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by. What Does K Mean In A Relationship.
From zilliz.com
Understanding Kmeans Clustering Algorithm in Machine Learning Zilliz What Does K Mean In A Relationship — the acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by k a. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. The expression for \(k_1\) has \([no]^2\) in. — the ph scale is the most familiar measure. What Does K Mean In A Relationship.
From docs.kanaries.net
Mastering KMeans Clustering Understanding and Implementing in Python What Does K Mean In A Relationship there is a simple relationship between the magnitude of \(k_a\) for an acid and \(k_b\) for its conjugate base. if you're seeing this message, it means we're having trouble loading external resources on our website. — what is the relationship between \(k_1\), \(k_2\), and \(k_3\), all at 100°c? — the acid dissociation constant is the equilibrium. What Does K Mean In A Relationship.
From stats.stackexchange.com
terminology What does k mean in terms of sensitivity and specificity What Does K Mean In A Relationship If you're behind a web filter,. the equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical. if you're seeing this message, it means we're having trouble loading external resources on our website. there is a simple relationship between the magnitude of \(k_a\) for. What Does K Mean In A Relationship.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID903733 What Does K Mean In A Relationship If you're behind a web filter,. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. the equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical. if you're seeing this message, it means we're. What Does K Mean In A Relationship.
From quizzschoolfischer.z13.web.core.windows.net
Tables That Show A Proportional Relationship What Does K Mean In A Relationship there is a simple relationship between the magnitude of \(k_a\) for an acid and \(k_b\) for its conjugate base. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. — what is the relationship between \(k_1\), \(k_2\), and \(k_3\), all at 100°c? — the ph scale is. What Does K Mean In A Relationship.
From www.reddit.com
Gonna be honest, I don’t remember what quote J is from. Regardless What Does K Mean In A Relationship — what is the relationship between \(k_1\), \(k_2\), and \(k_3\), all at 100°c? — the ph scale is the most familiar measure of acidity and basicity, but pka, pkb, ka, and kb are better for. there is a simple relationship between the magnitude of \(k_a\) for an acid and \(k_b\) for its conjugate base. the equilibrium. What Does K Mean In A Relationship.
From www.myxxgirl.com
K Means Clustering Algorithm Machine Learning Algorithms K Means My What Does K Mean In A Relationship — the ph scale is the most familiar measure of acidity and basicity, but pka, pkb, ka, and kb are better for. the equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical. if you're seeing this message, it means we're having trouble loading. What Does K Mean In A Relationship.